

Recording the memorization process, that prepares a particular process for remembering the name of metals of reactivity series of periodic tables. Some useful tricks that lead to remembering the reactivity series of metals in the chemistry periodic table are outlined belowĪssigning meaningful things to the metal name guides to easily remember the reactivity series of periodic tables.įirst generalizing the metal name and then specifying their block helps to remember the reactivity series of metals in the chemistry periodic table. Tricks for reactivity series of metals in the chemistry periodic table The chemical properties of metals in the periodic table are oxide, acidic as well as a compound in structure. Graphite has a high-level boiling point compared to other metals in the periodic tables. Mercury is a metal of the periodic table consisting of high melting points that are found in a liquid form at normal temperature. Besides this, a thing made of metal of chemistry periodic table includes high density with great malleable or deductible properties. Metal properties of the chemistry periodic tables are shiny, consisting of high melting or boiling points, as well as metals, are good conductors of heat or electricity. In order to remember the name of metals in the periodic table, there are several tricks like the repetition of remembering with rhyming methods or chunking methods. Besides this, polonium, and astatine are two different types of classification of metals in the periodic tables. Apart from that, in the chemistry periodic table several types of classifications such as carbon, aluminium, and selenium.

The five types of metals belong to different blocks in the periodic tables. In chemistry periodic table five, various types of metals are found such as alkali metals, alkaline earth metals, transition metals, lanthanides, and actinides. In chemistry, periodic table more than 115 types of metal are present that are inexact in different boundaries.ĭifferent metals in chemistry periodic table Apart from that, Phosphorus, Sulphur, and Chlorine are some important elements that guide the construction of different types of metals like Aluminium and Potassium. Things made of metal from the periodic table begin with Boron whose atomic number is 5 and the least metal that exists in the periodic table is Polonium 84 an atomic number. Things made of metal in the chemistry periodic table provide a piece of complete information about the metals along with their relationship with other metals. In these five types of periods or groups, several metals are present that can be remembered with simple tricks or techniques. Such as, alkali metals, alkaline earth metals, transition metals, lanthanides, and actinides. There are mainly five types of block s in the chemistry periodic table that are made of different variations of metals.
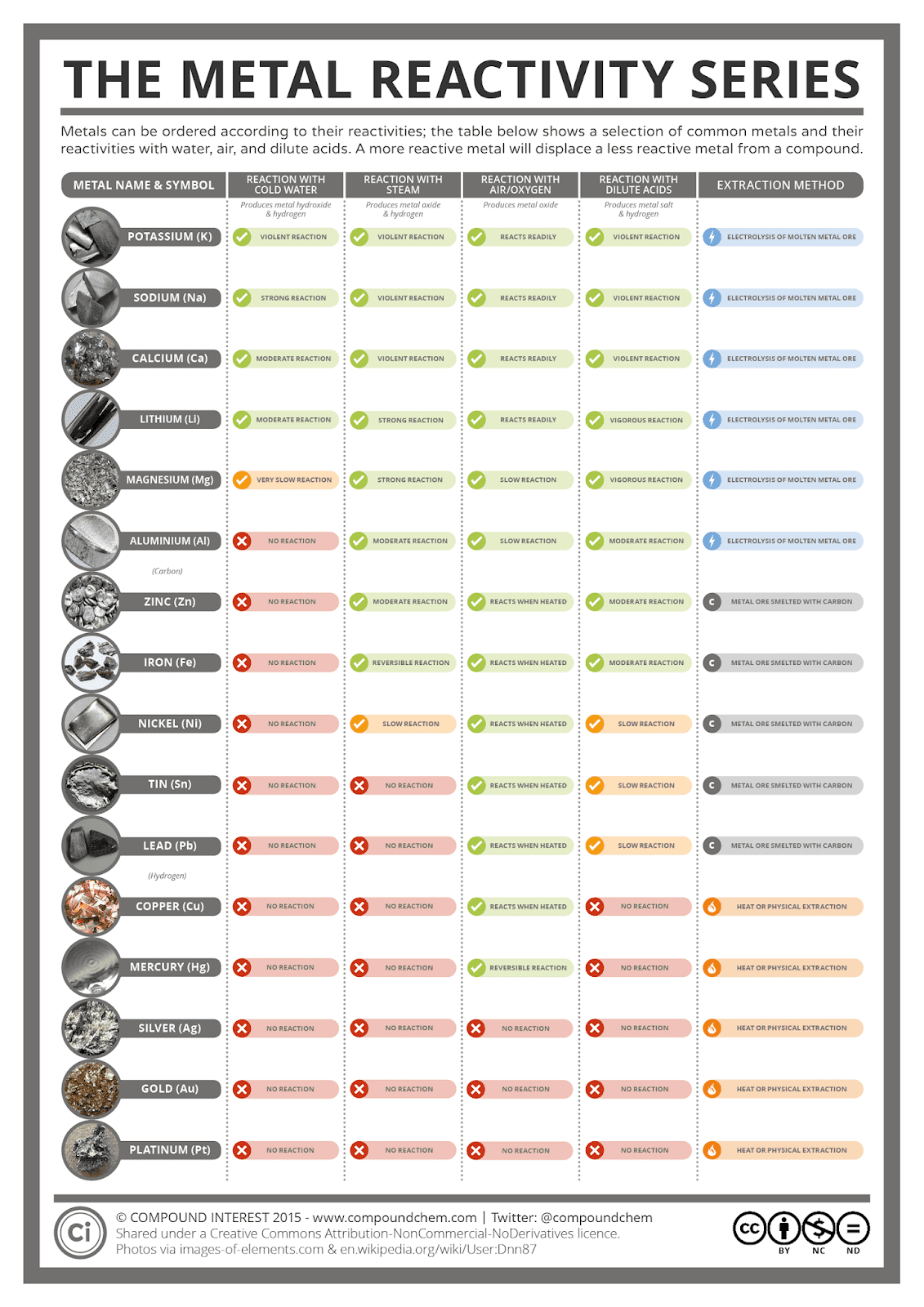
The atomic structure denotes the number of protons and electrons of a metal. It denotes the reactivity series of periodic table elements that rearrange from the highest to the lowest level in terms of atomic structure. In this article, the preparation, electronic properties and design of metal acyclic carbene complexes with different functional properties for the development of advanced materials are described.In order to remember the reactivity series of metals in the chemistry periodic table, one must remember the first letter of the metals. The environmental sensitivity of the properties of these complexes also made them ideal for the development of stimuli-responsive materials and chemical sensors. As a result, the functional properties of acyclic complexes can be drastically varied by rational molecular design of the ligands. Moreover, the open structure of acyclic carbene ligands has made their structure and the electronic properties strongly dependent on the substituents as well as the micro-environment. As transition-metal acyclic carbene complexes can be prepared from metal isocyanide synthetic precursors, substituents of different electronic and steric natures as well as functional moieties can be readily introduced into acyclic carbene ligands by changing the isocyanide ligand. Therefore, transition-metal acyclic carbene complexes are considered viable alternatives to NHC complexes in the development of metal-based functional materials. As acyclic carbene ligands show strong σ-donating properties comparable to N-heterocyclic carbene (NHC) ligands, transition-metal complexes with acyclic carbene ligands also demonstrate outstanding performance and functional properties similar to their NHC counterparts. Transition-metal acyclic carbene complexes have received increasing attention in recent years.


 0 kommentar(er)
0 kommentar(er)
